stay connected!
Fill in the required fields to stay up to date with news and announcements about VPRIV.
*Required Field
thank you!
You have been successfully signed up to receive
communications from Takeda
Click here to unsubscribe
HOW ERT WORKS & VPRIV MOA



HOW ERT WORKS
- Glucocerebrosidase deficiency causes the build-up of lipids in the lysosomes, leading to the formation of Gaucher cells4
- Over time, these Gaucher cells infiltrate various organs, particularly the spleen and liver, resulting in a progressive, heterogeneous disease4
- ERT is an infusion that replaces or supplements the deficient glucocerebrosidase enzyme2,3
ERT replaces or supplements deficient glucocerebrosidase.2,3
ERT IS A FIRST-LINE TREATMENT
- ERT is a first-line treatment for Gaucher disease that has been used for many years2
- ERTs address the underlying enzyme deficiency2,3
- Treatment with ERTs has been used for visceral debulking (reducing spleen and liver volumes) and improving hemoglobin and platelet counts2,3


VPRIV MECHANISM OF ACTION (MOA)
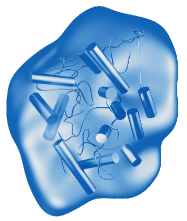
- VPRIV is a hydrolytic lysosomal glucocerebroside-specific enzyme indicated for long-term ERT for patients with type 1 Gaucher disease1
- VPRIV’s safety and efficacy were evaluated in the largest clinical trial program of an ERT for type 1 Gaucher disease; VPRIV was studied across three clinical trials (n=99; aged ≥4 years)1,5,6
VPRIV, an ERT
- VPRIV is produced using gene-activation technology in a human cell line1
- VPRIV has the same amino acid sequence as the naturally occurring human enzyme, glucocerebrosidase1
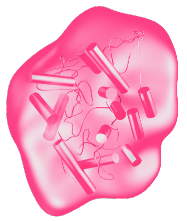
VPRIV is specifically designed to match and replace the natural human enzyme.1,7
Glucocerebrosidase
VPRIV
VPRIV’s glycosylation pattern is intended to facilitate targeted uptake into macrophages.*1,7–9
VPRIV Mechanism of Action Video
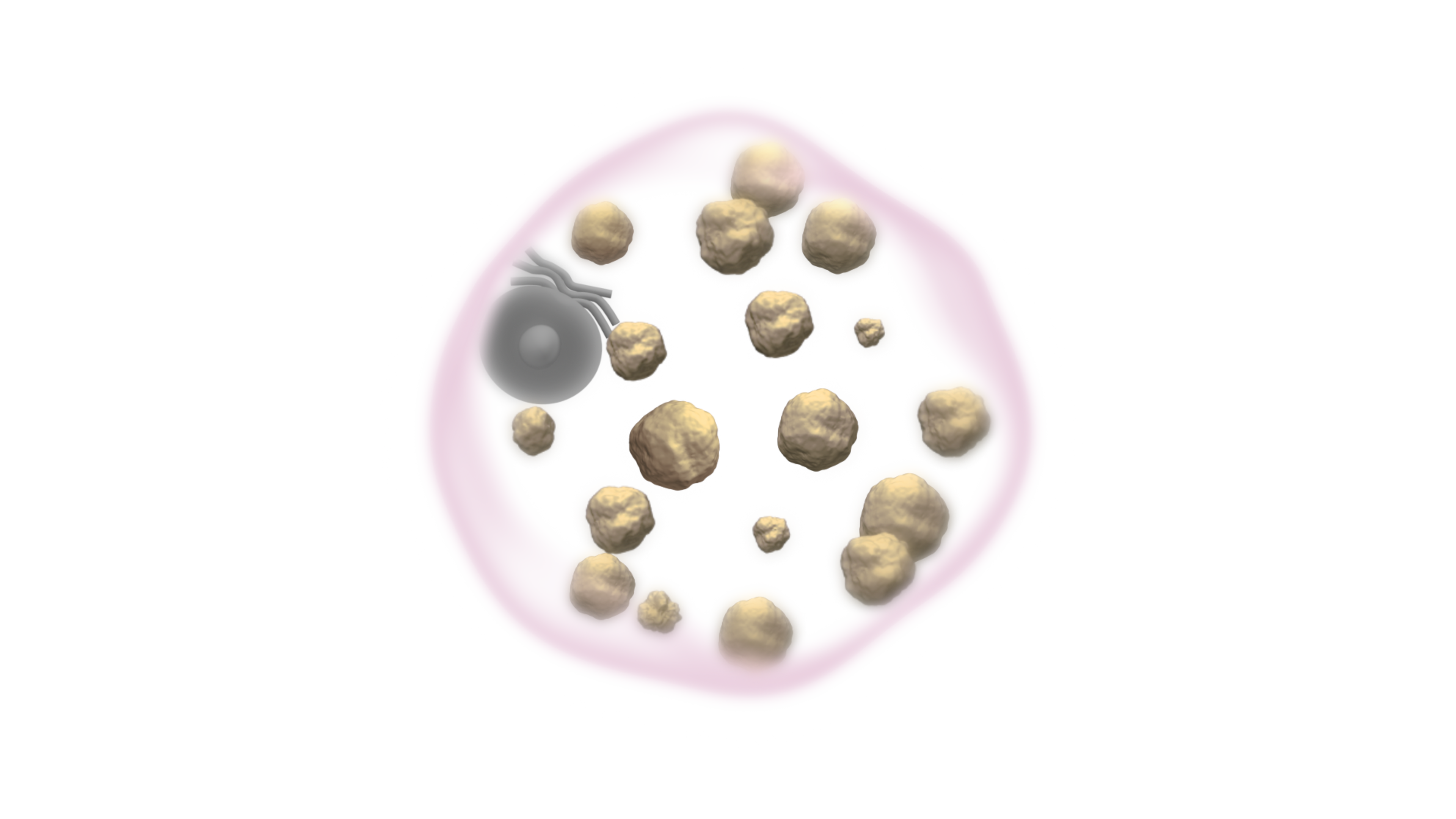
Gaucher cells are swollen due
to the accumulation of a fatty substance known as glucocerebroside.10,11
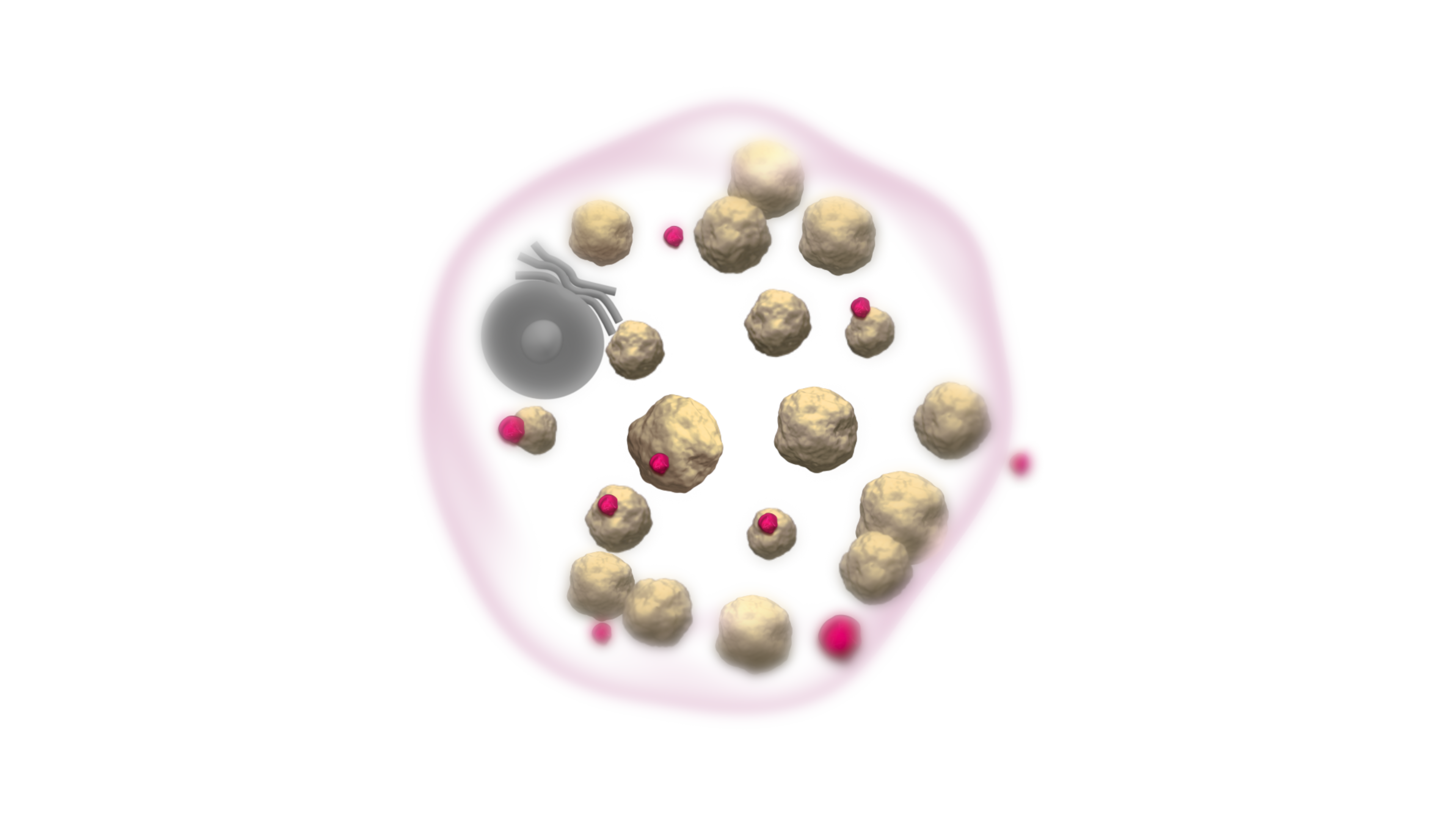
VPRIV is designed to bind to, and be absorbed into, the cell. Once inside the cell, VPRIV breaks down glucocerebroside.1
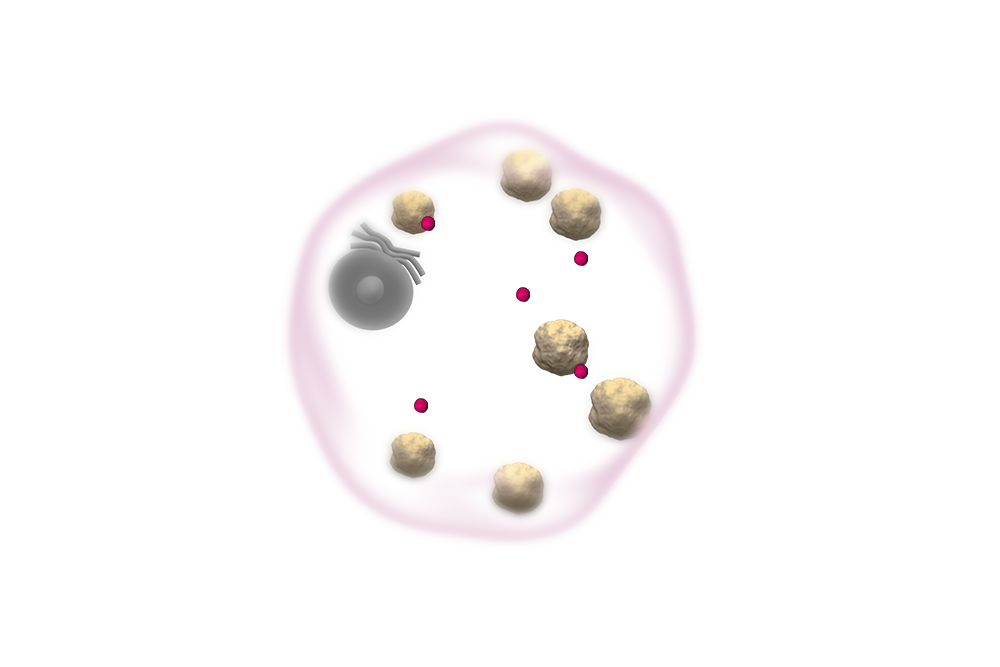
Like the naturally occurring human enzyme, VPRIV breaks down glucocerebroside, reducing the overall amount in the cell.1
*For illustration purposes only. In vitro test results do not necessarily correlate with clinical efficacy
IMPORTANT SAFETY INFORMATION
WARNING: HYPERSENSITIVITY REACTIONS INCLUDING ANAPHYLAXIS
Patients treated with enzyme replacement therapies have experienced life-threatening hypersensitivity reactions, including anaphylaxis. Anaphylaxis has occurred during the early course of enzyme replacement therapy and after extended duration of therapy.
Initiate VPRIV in a healthcare setting with appropriate medical monitoring and support measures, including access to cardiopulmonary resuscitation equipment.



